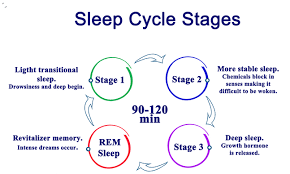
The human sleep cycle2025: represents a highly orchestrated and meticulously regulated neurophysiological process, typically spanning 90 to 110 minutes per iteration. This cyclic progression through distinct sleep stages is integral to cognitive restoration, emotional regulation, metabolic homeostasis, and systemic equilibrium. Contemporary research in sleep medicine and cognitive neuroscience continues to illuminate the profound impact of sleep architecture on neurological resilience, psychiatric stability, cardiovascular integrity, and immunological function. Given its intricate interplay with neurobiological mechanisms, sleep cycle disruptions have been increasingly implicated in neurodegenerative pathologies, metabolic disorders, and psychiatric conditions, necessitating an advanced understanding of its mechanisms and clinical significance. The advent of cutting-edge neuroimaging techniques and molecular biomarker research has facilitated a more nuanced appreciation of the bidirectional relationship between sleep and overall health, further reinforcing the role of sleep as a cornerstone of systemic homeostasis.
Neurophysiological Underpinnings of the Sleep Cycle
The sleep cycle consists of sequential transitions between non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, each distinguished by unique neurochemical profiles and cortical activity patterns. These phases are regulated through a complex interplay of GABAergic inhibition, cholinergic excitation, and serotonergic modulation, which together maintain the oscillatory balance between sleep depth and wakefulness. The neurophysiological regulation of these states also involves intricate feedback loops within the hypothalamic-pituitary-adrenal (HPA) axis, modulating stress resilience, emotional stability, and memory processing during sleep.
1. NREM Sleep: Transition to Deep Restorative States
- Stages 1 & 2 (Light Sleep): These initial phases serve as a bridge between wakefulness and deep sleep, characterized by progressive cortical deactivation, autonomic attenuation, and thalamocortical gating. Thalamocortical oscillations regulate sensory disengagement, synaptic pruning, and attentional recalibration, contributing to cognitive recovery. The interaction between thalamic relay neurons and cortical inhibitory circuits orchestrates the suppression of sensory input, preventing unnecessary arousal during these early sleep stages.
- Stage 3 (Slow-Wave Sleep, SWS): Characterized by high-amplitude, low-frequency delta oscillations, extensive cortical inhibition, and peak glymphatic clearance, SWS is vital for synaptic homeostasis, neurotoxic metabolite clearance, and neuroendocrine regulation. The attenuation of SWS with age is directly correlated with cognitive decline and increased susceptibility to neurodegenerative conditions such as Alzheimer’s disease. Recent studies suggest that compromised glymphatic flow during SWS may exacerbate amyloid-beta accumulation, linking sleep disruption to neurodegenerative progression.
2. REM Sleep: Neurocognitive and Emotional Integration
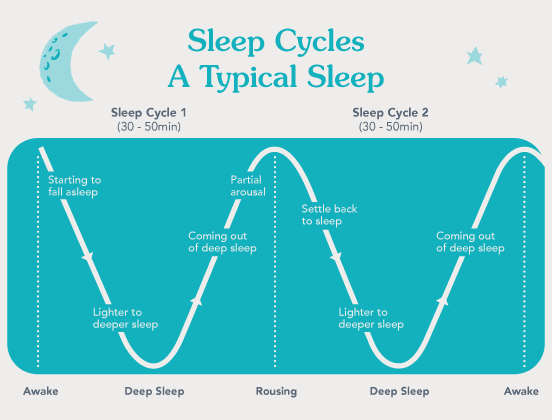
- Characterized by desynchronized cortical activity, rapid eye movements, transient muscle atonia, and episodic autonomic fluctuations.
- Essential for memory reconsolidation, synaptic plasticity, and emotional recalibration, predominantly mediated through heightened activity in the amygdala, hippocampus, and prefrontal cortex.
- REM sleep duration progressively increases across successive sleep cycles, with perturbations in REM homeostasis linked to mood dysregulation, cognitive inflexibility, and increased psychiatric vulnerability.
- Alterations in REM latency and fragmentation serve as potential biomarkers for psychiatric conditions, including major depressive disorder (MDD), post-traumatic stress disorder (PTSD), and schizophrenia. Advanced neuroimaging modalities have further elucidated the role of REM sleep in emotional resilience, implicating dysfunctions in limbic circuitry as potential therapeutic targets for mood disorder interventions. Read more
Chronobiological Modulation and Variability Across the Lifespan
Sleep cycle architecture is subject to modulation by both endogenous circadian rhythm regulation via the suprachiasmatic nucleus (SCN) and exogenous factors such as light exposure, feeding schedules, and socio-behavioral influences. Across different life stages, notable shifts in sleep structure are observed:
- Neonates and Infants: Exhibit polyphasic sleep patterns, high REM sleep proportions, and underdeveloped circadian rhythmicity.
- Adolescents: Characterized by delayed sleep phase syndrome (DSPS) due to circadian shifts and heightened neurodevelopmental activity.
- Elderly Populations: Experience attenuated SWS, increased sleep fragmentation, and phase-advanced circadian timing, frequently manifesting as sleep maintenance difficulties and reduced sleep efficiency.
- Shift Workers and Night Shift Employees: Exposure to irregular light-dark cycles disrupts melatonin secretion, circadian entrainment, and metabolic stability, predisposing individuals to chronic health conditions such as obesity, insulin resistance, and cardiovascular dysfunction.
Pathophysiological Implications of Sleep Cycle Dysregulation
Aberrations in sleep cycle integrity are robustly associated with a spectrum of neurological, metabolic, and psychiatric disorders, including:
- Cognitive Impairment: Fragmentation of SWS and REM sleep results in deficits in executive function, working memory, and attentional processing.
- Mood Dysregulation: REM sleep abnormalities are strongly implicated in bipolar disorder, generalized anxiety disorder (GAD), and major depressive disorder (MDD).
- Neuroimmune Dysregulation: Chronic sleep deprivation elevates proinflammatory cytokine activity (IL-6, TNF-alpha, CRP), exacerbating systemic inflammation and immune dysfunction.
- Metabolic Dysfunction: Sleep deficits precipitate leptin resistance, ghrelin dysregulation, insulin insensitivity, and adiposity accrual, heightening the risk of obesity and type 2 diabetes.
- Cardiovascular Pathologies: Sleep disturbances alter nocturnal blood pressure regulation and endothelial function, contributing to an elevated risk of hypertension, arrhythmias, and myocardial infarctio
- https://todynew.in/
The Human Sleep Cycle 2025:
Cutting-edge research has led to the development of novel interventions aimed at optimizing sleep efficiency and circadian alignment, including:
- Genetically-Informed Sleep Medicine: Identification of polymorphisms in clock genes (PER2, CRY1, BMAL1) as biomarkers for sleep disorders and individualized treatment approaches.
- Pharmacogenomic Interventions: Personalized medication regimens based on metabolic and genetic profiling to optimize hypnotic efficacy while minimizing adverse effects.
- Technological Innovations: Development of AI-driven wearable sleep monitors and polysomnography enhancements to provide real-time analytics for clinical and home-based sleep assessments.
- Neurostimulation Therapies: Emerging research into transcranial magnetic stimulation (TMS) and vagus nerve stimulation (VNS) as potential therapeutic modalities for sleep disturbances and circadian misalignment.
Conclusion
The human sleep cycle is an evolutionarily conserved, neurophysiologically essential mechanism that governs cognitive function, metabolic stability, and emotional well-being. The maintenance of sleep cycle homeostasis is paramount for long-term neuroplasticity, systemic resilience, and psychiatric health. Advancements in chronobiology, neuropsychiatry, and precision sleep medicine continue to refine our understanding of sleep, underscoring the necessity for biomarker-driven diagnostics, individualized sleep interventions, and pharmacogenomic innovations to optimize sleep outcomes and mitigate sleep-related pathophysiology. Future research must explore epigenetic modifications, sleep-microbiome interactions, and personalized neuromodulation strategies to further elucidate the intricate interplay between sleep and human health.
Genetically-Informed Sleep Medicine: Identification of polymorphisms in clock genes (PER2, CRY1, BMAL1) as biomarkers for sleep disorders and individualized treatment approaches.